23rd, April 2019
Protein Alternatives S.L. (PROALT) has begun the clinical validation study of its blood-based diagnostic test for colorectal cancer in Luminex® and ELISA-based formats. COLODETECT® test is based on detecting in blood the autoantibodies (AAbs) generated by the human body against proteins related to the colorectal cancer tumor, known as tumor-associated antigens (TAAs) or neoantigens. The simplicity of COLODETECT® will allow the screening of large population at risk.
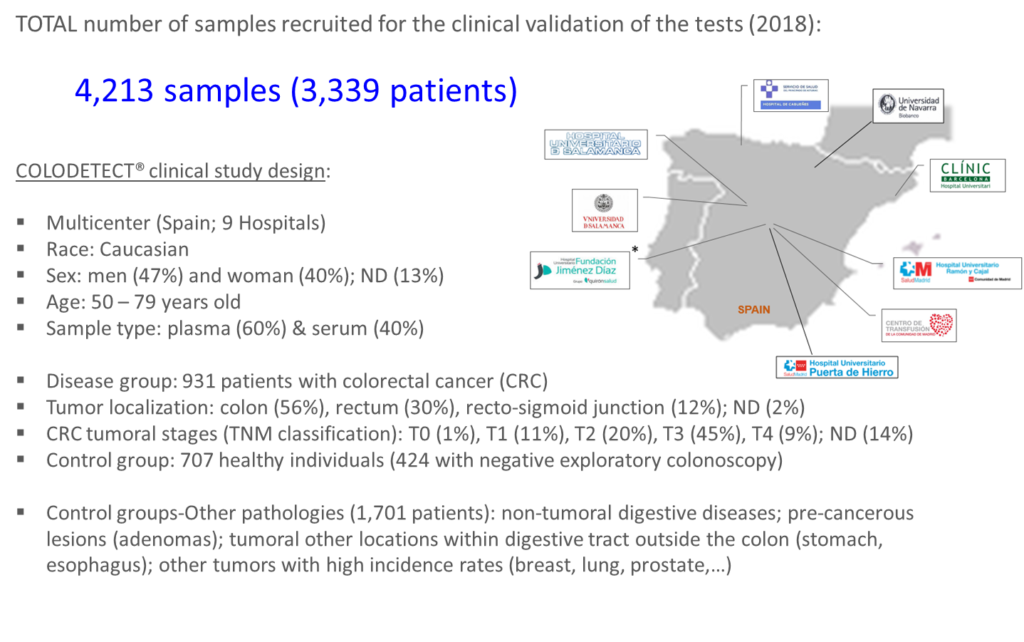
More than 4,200 samples were collected from nine different hospitals in Spain.
 Patient’s samples will be tested with the prototypes developed:
Patient’s samples will be tested with the prototypes developed:
- ELISA-based format (enzyme-linked immunosorbent assay).
- Luminex® xMAP® format (multiplex bead-based assay).
We are right in our schedule with the validation study and are confident that we will be able to launch COLODETECT® onto the market as planned at the end of 2019 beginning of 2020.
About Protein Alternatives:
Founded in 2006 and based in Tres Cantos city (Madrid, Spain) ProAlt is a biotechnology company focused on the development and commercialization of (i) biomarker-based assays for cancer diagnosis and clinical management and (ii) therapeutic monoclonal antibodies against novel targets aimed for the treatment of highly aggressive colon, breast and melanoma tumors. The company also offers its (iii) own manufactured innovative recombinant proteins and antibodies, and different custom-tailored integral services in protein design, expression and purification. Service offer is performed under individual customer contracts (CRO/CMO) to meet customers needs.